Supraglottic Device Change
As you are aware, we have recently completed a field trial with our first responder partners with the Charlotte Fire Department of a different supraglottic airway device (SGD).
Based on the results of that trial and additional reviews of the product, we will be changing our supraglottic device to the i-gel®. Results of that trial did not show any difference between the i-gel® and the King-LTTM with respect to airway adverse events, first time insertion success rates, need for intubation following SGD attempt, or field sustained field ROSC achieved. Ease of use however, was significantly more often rated as very easy or easy for the i-gel® vs. the King-LTTM.
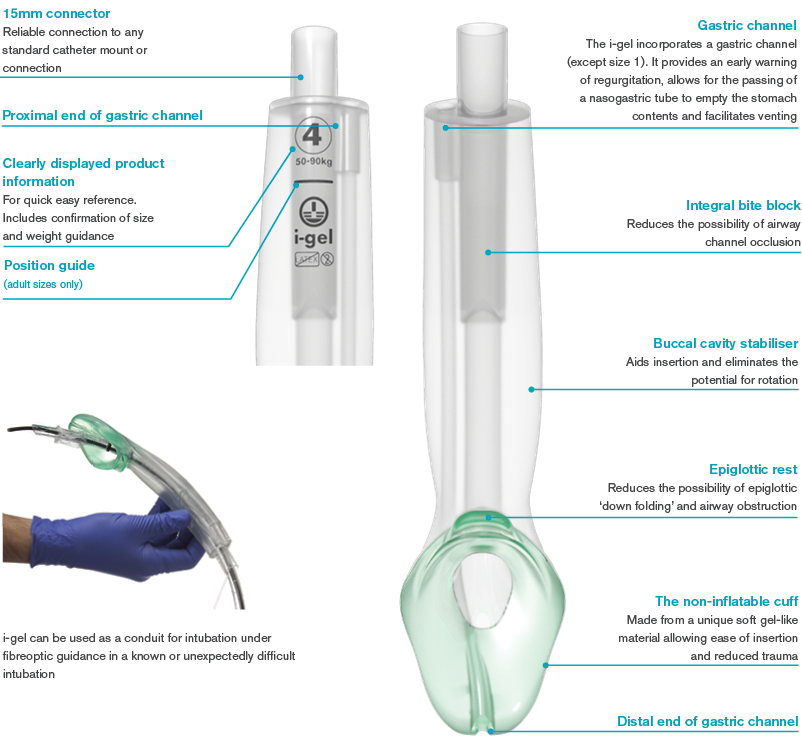
The i-gel® has a soft, gel-like, non-inflatable cuff, designed to provide an anatomical fit over the laryngeal inlet. i-gels are supplied in a color-coded protective cradle (3 adult sizes) or cage pack (4 pediatric sizes).
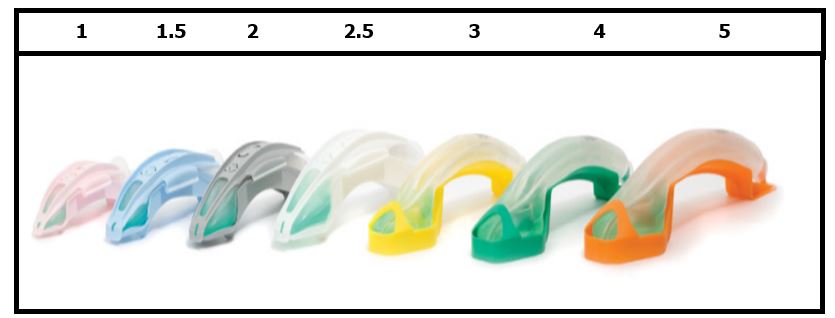
Both Medic and our first responders will be utilizing all sizes of the i-gel®. Training has been developed and is being delivered to all first responder agencies and will be provided to Medic personnel.
The i-gel® has several benefits compared to the King-LTTM including no balloon required to be inflated, and once delivered to the emergency department, the patient can be transitioned to an endotracheal tube through the i-gel® vs. having to remove the SGD prior to performing intubation.
Indications and contraindications are the same as with any SGD. Indications: cardio-respiratory arrest; contraindications: responsive patients with an intact gag reflex, known oropharyngeal disease, caustic substance ingestion.
As other SGDs, there is a limit to the peak airway pressure that the i-gel® can support (40 cm H2O). SGDs are limited in patients with significantly airway pressures.